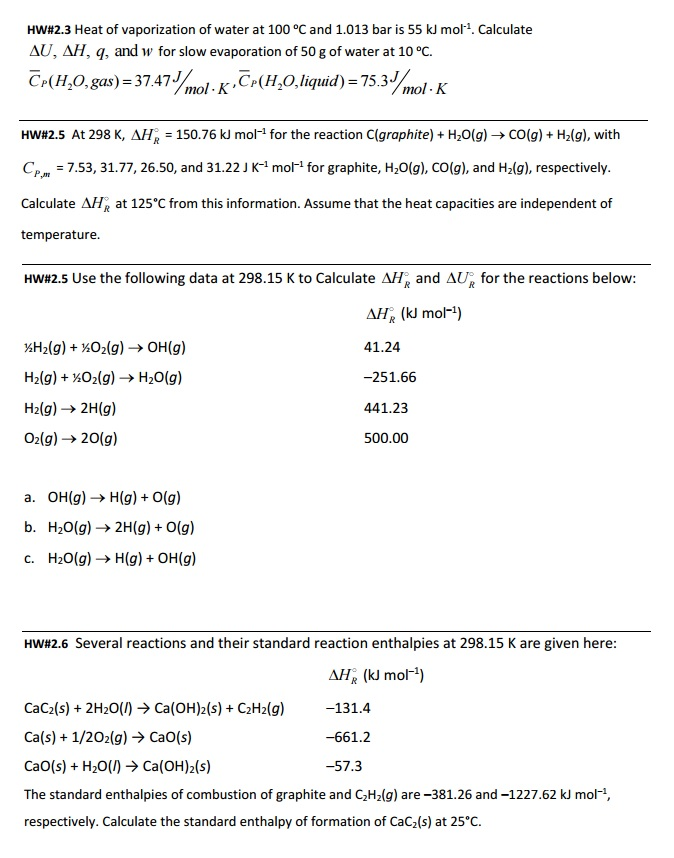
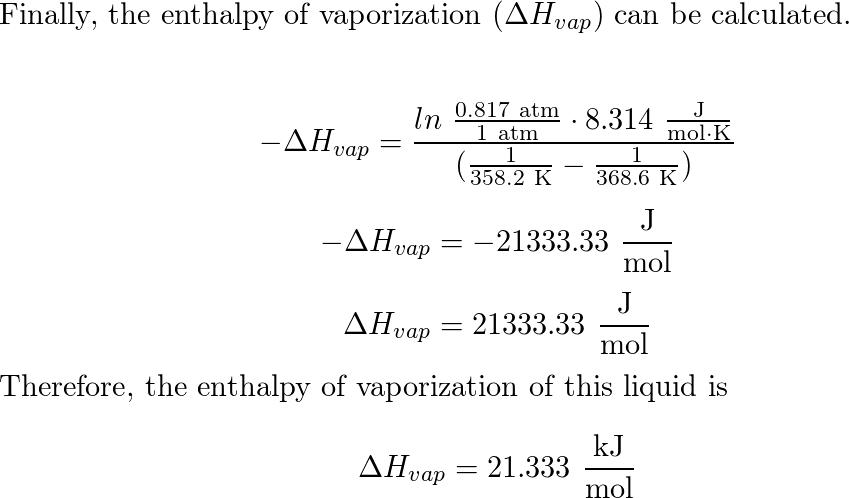SOLVED: For rubidium ∆H°vap = 69.0 kJ/mol at 686°C, its boiling point. Calculate w and ∆E for the vaporization of 1.00 mol rubidium at 686°C and 1.00 atm pressure. w = ?kJ ∆E = ?kJ
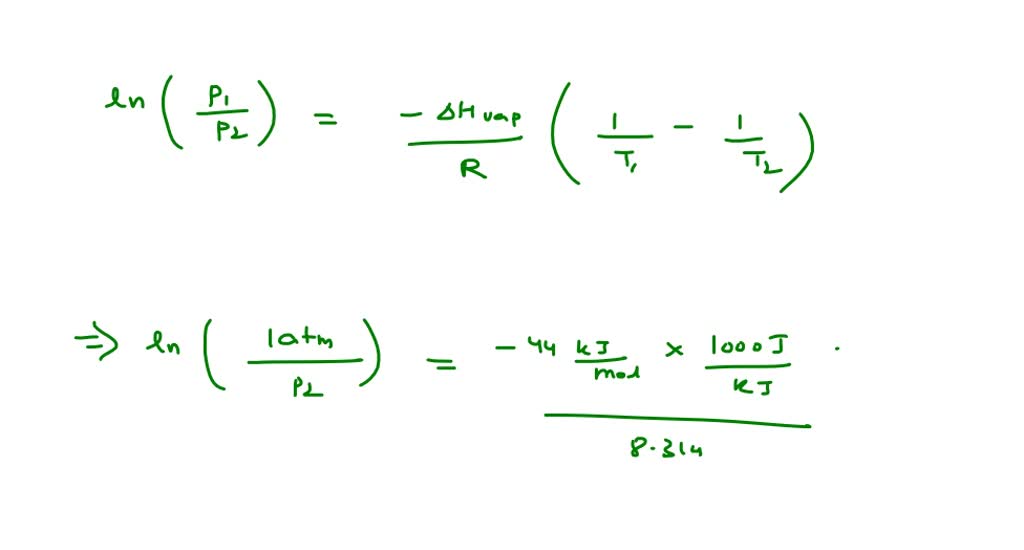
SOLVED: The standard enthalpy of vaporization (H°vap) of ethylenediamine ((NH2CH2)2) is 44.0 kJ/mol. The normal boiling point of ethylenediamine is 116.5°C. Calculate the vapour pressure of ethylenediamine at 95.0°C.
88. The values for delta H vap.and delta S vap. for ethanol are respectively 38.594 kJ/mol and 109.8 J/K. What will be the boiling point of ethanol ?

Heat of vaporization ∆H vap as a function of temperature at pressure... | Download Scientific Diagram
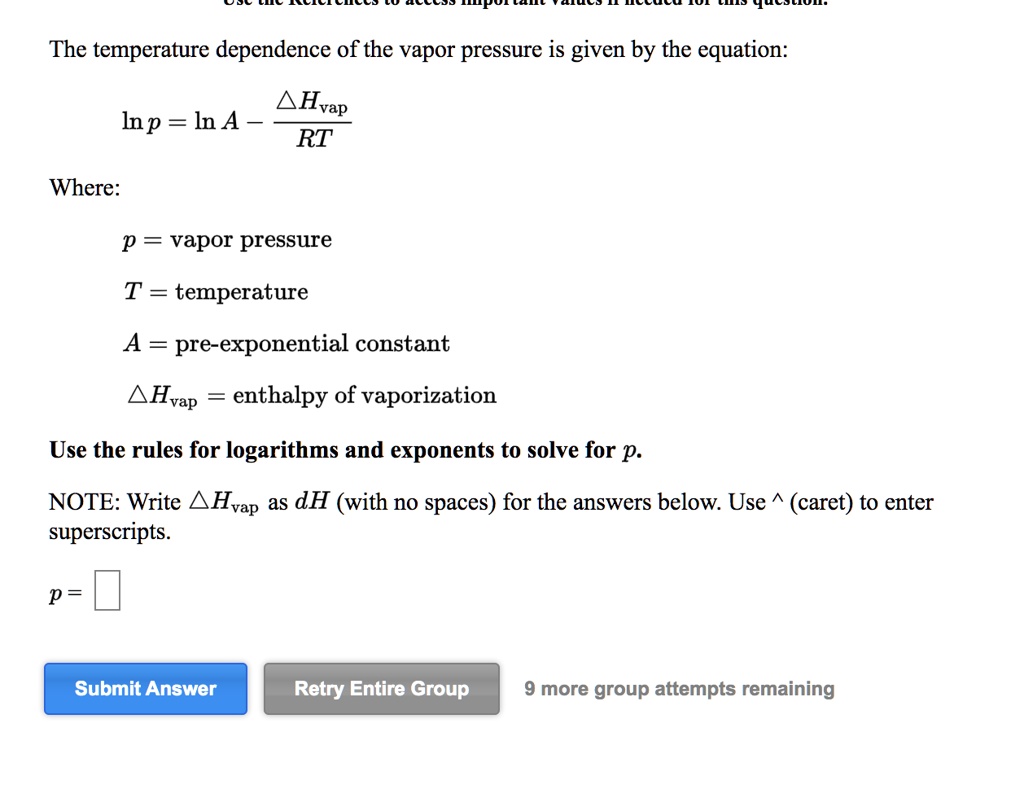














-438.png)